
Food | Decaffeination of coffee beans | Measurement of caffeine in dichloromethane
Precise determination of residual caffeine is important to rule out health risks for consumers
More and more consumers choose to reduce or eliminate their caffeine consumption. As a result, decaffeinated coffee is forecast to experience a significant market growth of 7 % per year through to 2027. While for some people the stimulating effect of caffeine is the primary reason for this decision, for others the consumption of caffeinated coffee can cause serious damage to health. These include people with a true caffeine intolerance, heart problems, high blood pressure, a sensitive stomach, and those with pre-existing conditions such as cirrhosis of the liver or cardiac arrhythmias. Pregnant and breastfeeding women are also advised to reduce their caffeine intake, as it can affect fetal growth.
These groups of people must be confident that the declared caffeine-free coffee they consume does not in fact exceed the EU legal limit for residual caffeine of 0.1 %. Measuring the caffeine content in both the green coffee bean and the solvent blend after extraction is therefore a critical and quality-decisive process step that must be monitored precisely and in real time.
The decaffeination of green coffee beans is subject to a complex handling process. The industrial process involves pre-moistening the beans with water, caffeine extraction and subsequent drying of the beans.
Coffee is a natural product, and the caffeine content in the beans can vary depending on the harvest. In addition, coffee beans contain a variety of other substances besides caffeine, which can make it difficult to determine low concentrations of caffeine. There is a risk that other aroma-imparting substances will be removed from the bean. Ideally, when the bean is decaffeinated, the caffeine is removed from the cells without altering them in any way. This prevents undesirable side effects such as loss of aroma, changes in the structure and size of the beans, or solvent residues.
In conventional manufacturing processes, the caffeine content of the coffee bean is only determined at the beginning and end of the decaffeination process. Any deviations from the threshold for residual caffeine content in the coffee bean are thus only detected at a very late stage. Reliable control of the process and prediction of the yield is therefore only possible to a limited extent.
A frequently used method for decaffeinating green coffee beans is the extraction with dichloromethane (DCM), as this can very selectively dissolve caffeine from the beans even at low temperatures, so that all other aroma substances of the coffee remain almost untouched in the coffee bean.
In this type of decaffeination, the green coffee beans are first brought into contact with steam and water to increase their moisture content from about 10 % to up to 40 % of their weight, thus making their structure accessible to the solvent. Subsequently, the water-swollen beans are decaffeinated by extraction with the organic solvent either in a fixed bed (e.g., percolating column batteries, carousel extractors) or in agitated systems (e.g., rotating drums).
The content of caffeine in the solvent mixture is then measured spectroscopically to verify that the caffeine has been completely extracted from the beans. Finally, the remaining dichloromethane solvent is removed from the beans by steaming and the beans are dried again so that they regain their original moisture content.
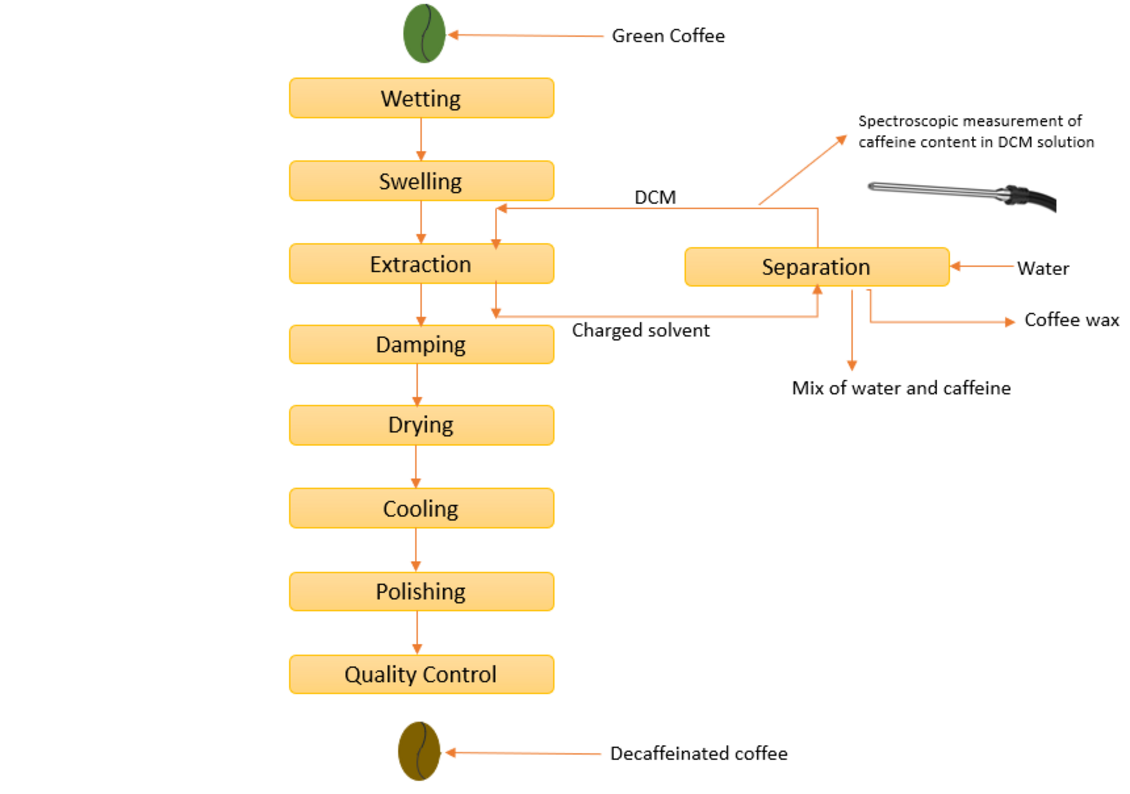
The caffeine extracted from the beans in the DCM solution can be detected spectroscopically. The spectra recorded with the aid of an optical immersion probe show that the absorption decreases the less caffeine there is in the solution.
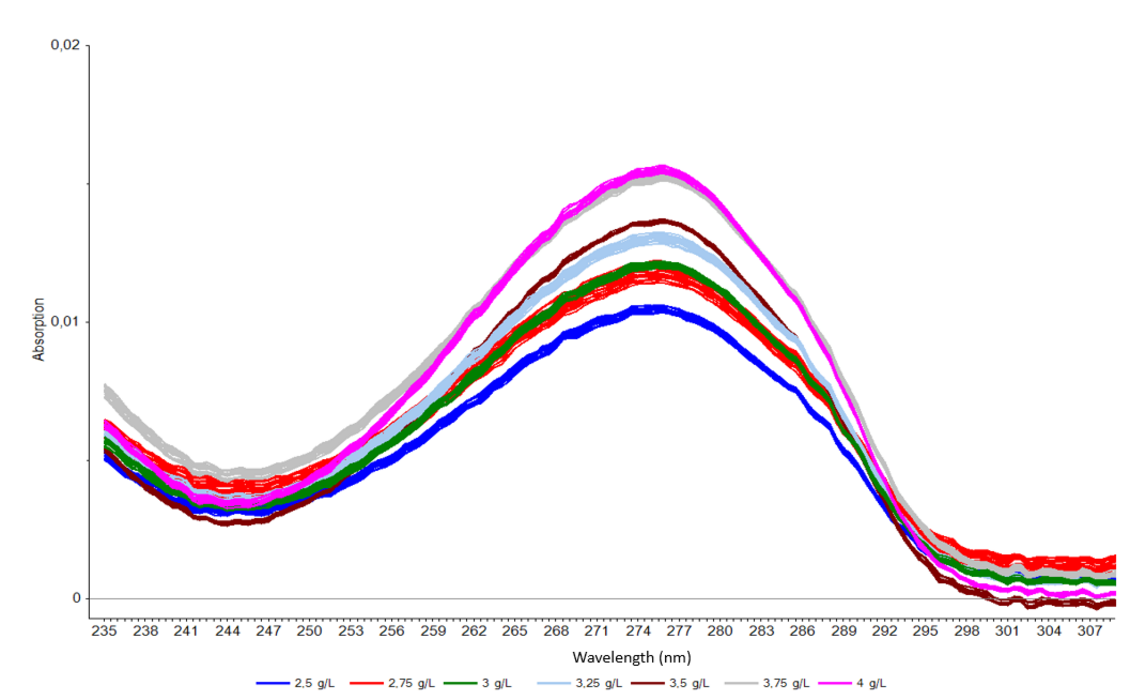
Spectra of seven DCM solutions with different caffeine concentrations
However, due to the high optical density of caffeine, a transmission measurement is unsuitable for this application. A better method for strongly absorbing media is the ATR (Attenuated Total Reflection) measuring principle based on the principle of total internal reflection. The medium to be measured flows around the prism of the probe and the measurement signal is attenuated at each reflection at the boundary layer between the medium and the prism. For high measurement precision with these media, Hellma provides the ATR probe »Katana XP«. This immersion probe features a sapphire crystal with 3 total internal reflections. The light is almost completely reflected on the inside of the ATR crystal, with the solution in contact with the crystal absorbing a small part of the light on the surface of the crystal.
Because the absorption occurs only at the surface of the crystal, the equivalent path length is extremely small, only approx. 1 µm. This makes the Hellma ATR Katana immersion probe particularly suitable for direct measurements in strongly absorbing solutions where standard transmission probes cannot be used.
For integration of the probe in the process, a bypass is placed at the reaction vessel with the solution to be measured and the online measurement is realized there. The information is then transmitted directly from the bypass to a UV spectrometer by means of quartz optical fibers.
For integration of the probe in the process, a bypass is placed at the reaction vessel with the solution to be measured and the online measurement is realized there. The information is then transmitted directly from the bypass to a UV spectrometer by means of quartz optical fibers.
Using online measurement technology at critical process stages, close-meshed process monitoring and control is possible. With the help of accurate and reliable measurement data, a high level of process reliability and ultimately a higher yield as well as very good product quality is achieved. Due to its compact design, the probe can be easily integrated into the process.
